Understanding Cancer
Understanding lung cancer
Many people facing a diagnosis of lung cancer want to know more about their disease. Like all cancers, lung cancer has remained a mystery for centuries; only recently have we been able to understand its biology and behavior.
What is cancer?
There is no simple answer to this question. Anyone who has Googled this question on their computer has discovered this fact for themselves. The fact that there are so many wacky websites offering ridiculous explanations for cancer (and promoting scam cures) is testimony that it is difficult to describe or explain what cancer really is. If the answer to the question: “What is cancer?” were easy, then there would be no room for the outlandish answers and supposed cures on the internet.
Cancer is a disease of DNA. It is THE disease of DNA.
The fundamental problem in cancer is the loss of normal DNA regulation and control. DNA is a loaded gun, or a leaking gas stove, or a piece of glass in the sand. It is dangerous stuff. It contains enormous power and potency. It can create a heart, or a liver, or a brain, or a foot, or a head; it contains the information to guide the growth of an embryo into an infant child into a fully grown, mature adult. But each individual cell only uses a fraction of this information. Cancer is what happens when a cell starts to use DNA information that it isn’t supposed to. In order for the human body to function normally, DNA HAS to be highly controlled or regulated. It is the breakdown of this regulation of DNA that causes cancer.
DNA is usually safeguarded by the cell. It is protected in its own cellular compartment, the nucleus, and wrapped in its own insulation, the histone proteins. It is constantly monitored and repaired. If it breaks, it is precisely put back together. If it gets irretrievably damaged, the cell undergoes programmed suicide or “apoptosis” in order to prevent the damaged DNA from propagating and replicating. The DNA in cancer cells loses this protective shield. Gradually at first, and as the cancer cells mutate, the loss of regulation speeds up until the system gets reduced to a jumble of DNA and non-functioning or poorly functioning regulation. Gene programs get turned on and off, accelerating the mutational chaos. This mess of genetic disinformation gets expressed on the tissue level as a tumor, and eventually, spread to other parts of the body as metastasis. The very fact that cancer can tolerate this loss of DNA regulation is evidence how deformed and foreign it is. Normal cells do not tolerate even the slightest abnormalities in DNA regulation that are routinely identified in cancer cells.
To give an example: normal cells will not tolerate an abnormal number of copies of chromosomes. Normal cells undergo apoptosis (programmed suicide) in this situation but in cancer cells, this regulatory limit has been undone and cells with double or even triple the number of normal chromosomes will continue to live and divide.
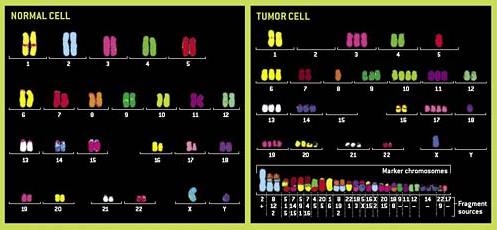
Figure 1. shows a graphic example and how abnormal the chromosomes in cancer cell can become. A depiction of chromosomes (the packaged form of DNA and genes) from a normal cell on the left is contrasted with the disordered and abnormal collection of chromosomes on the right in a cancer cell. Note the many fragments of chromosomes in the cancer cell, the duplication of chromosomes #10, 17, and 19, and complete deletion of chromosomes # 2 and 15.
The precise and elegant regulation of DNA that has been perfected over the course of hundreds of millions of years of biologic evolution gets undone and disrupted by cancer over the course of months to years.
Cancer is diseased regulatory DNA. We know that only 2% of DNA codes for proteins. While we haven’t yet assigned a role for the rest of DNA, we know that much of the functional DNA that doesn’t code for protein synthesis is involved in DNA regulation. There are thought to be around 25,000 protein coding genes in the human genome. The number of gene mutations discovered in the tumors studied so far ranges from dozens to hundreds.
In what cells does cancer originate in?
Cancer starts in tissue stem cells. Tissue specific stem cells are the cells that regenerate normal tissue and organ cells during routine cell turnover of tissue. Normal tissue cells do not divide once they are mature and are in fact programmed to die. For these reasons they are not thought to be the source for tumors. Tissue stem cells on the other hand are ideal candidates for carcinogenesis because they already possess a fundamental characteristic in common with cancer cells: they have unlimited reproductive potential. They divide throughout the lifetime of the individual. Furthermore, these tissue specific stem cells divide at rates that vary with the turnover of the cells in the organs they reside in. For instance, there are certain tissues in the body where we know the cells turnover at a fairly high rate: intestine, skin, and respiratory tree. These cells are exposed to the outside world and are constantly being shed and regenerated. The source of the new cells is tissue stem cells. In fact, if one plots tissues in which cell turnover is known from highest to lowest, it parallels the incidence of cancer by organ.
Cancer growth.
The threat from any cancer is usually related to spread of disease, known as metastasis. Almost all patients who die from cancer succumb to distant or metastatic spread of cancer. However, virtually every cancer no matter the site, is potentially curable in its early stage. With time, growth, and evolutionary change of the tumor, a transition from curable to incurable occurs. I call this the “transitional phase” and it can be easily characterized if one looks at the stage distribution for lung cancer.
The amount of growth required for a cancer to go from a single cell to a deadly metastatic tumor spread throughout the body is traditionally thought to require 40 doublings in size of the primary tumor. During this time frame, cancer cells acquire the ability to evade the normal cellular mechanisms in place to destroy their aberrant genetic mutations; separate from their “organ” or tumor mass; evade normal immunologic surveillance; enter the blood stream; lodge in distant organs; recruit supportive surrounding cellular and extracellular matrix and continue to grow. Normal tissue cells don’t do any of these things.
Virtually every characteristic of cancer that can be measured shows that prognosis is worse with growth: size of primary tumor, number of lymph nodes involved, number of lymph nodes stations, distance of lymph nodes from primary, doubling time of tumors…almost any geometric measurement of tumors that indicates growth correlates with a worse outcome. Why is this? What are the biologic underpinnings of “growth” and why is this such a negative sign?
The answer is that “growth” is equivalent to tumor cell evolution. We now know that tumor cells undergo evolutionary change roughly equivalent to biologic evolution. The main difference is the extremely compacted time frame of evolution in cancer cells. Biologic evolutionary change happens on a time scale--thousands to millions of years-- that is hard to imagine. Cancer evolutionary change on the other hand occurs on a time scale that is so grotesquely fast, that it is also hard to grasp.
As mutations accumulate, and tumor cells acquire the functional adaptations that allow for metastatic spread, the transition to incurable cancer is inevitable.
And unpredictable. It is not possible to estimate the time point at which a cancer gives rise to the deadly metastatic cell clones that lead to the ultimate death of the patient. This process is ineluctable: once it has started, it almost always leads to the death of the patient unless treatment is given.
How do we get cancer? How does cancer start?
All cancers involve a mutation in DNA. DNA mutation is a normal part of biologic life. DNA mutations occur randomly but at a steady rate. Mutation rates are so constant that they can be used to estimate divergence of species over the course of millions of years.
The vast majority of mutations in our DNA are of no consequence, or else lead to the death of the cell in which they occur. The human body has an extremely detailed set of programs for dealing with potentially harmful mutations that include repair programs, as well as programmed cell death responses.
Cancer cells lose these protective mechanisms so that mutation in cancer cells persist far more easily compared to normal cells. The progressive accumulation of mutations leads to gain and loss of cellular functions not normally seen in normal cells.
DNA mutation is random and purposeless. I once had a patient comment to me that cancer must be “stupid” since, it kills its “host”…...but this is exactly what purposeless mutation means, the “purpose” so to speak is determined by selection. This is the cruelest irony of cancer biology. Cancer develops and grows using the same principles of natural evolution we see in the world around us. And it does it on a biological time frame measured in days, months and years, rather than centuries or millennia that characterize biologic evolution.
Mutations are most likely to occur when DNA is copied during cell division. Therefore, anything that increases cell division will increase the opportunity for mutations, and eventually the possibility of a cancer initiating mutation.
The simplest explanation why humans get cancer is also the easiest to understand: human beings live longer than our biologic program. The name of the game in biology is to reproduce. Once animals reproduce, the clock starts to count down. The same is true for humans, except that we have mastered many of the illnesses that would have killed our ancestors, specifically infectious diseases. This means we are outlasting our biologic time clock and this prolongation of lifespan means more cell divisions, means more mutations, means greater possibility for a cancer-causing mutation.
Cancer cells frequently have multiple copies of whole chromosomes or fragments of chromosomes (see Figure 1) which means there is more DNA available to be mutated in cancer cells. So these first two explanations are relatively easy to describe: more time for mutations to occur, and more DNA for mutations to act on.
The remaining causes of cancer are classified into three broad categories: hereditary, environmental, and replicative (or stem cell divisions). “Hereditary” refers to genetic mutations and cancer risk that are genetically passed on from parents to their children. In essence, a cancer causing mutation gets passed on. Examples of this would include BRCA gene in breast cancer and FAP in colon cancer. “Environmental” is a broad category which includes exposure to known carcinogens; examples of this would include exposure to sun in melanoma patients, smoking in lung cancer patients, and HPV in head and neck cancer and cervical cancer. These risks increase directly the chance of mutation or increase cell turnover or both.
The last cause is related to the number of stem cell divisions within organs and tissues. This is nothing more than the rate at which worn out cells in an organ or tissue need to be replaced. Tissue stem cells are the source of these replacement cells but also are the cells of origin for cancer. So higher turnover of these cells will also predispose to cancer in these cells.
Cancer cells in tumors divide at a much higher rate than normal cells. This higher rate of division also gives rise to a higher chance of deleterious mutations.
Implications for cancer treatment.
What does this mean for treatment? Firstly, an understanding of the biologic complexity of cancer makes it clear why cure by means other than surgery is difficult and unreliable. Surgery remains the best hope for cure in lung cancer as it is for most solid tumors. Time is of the essence. Delays in appointments, testing, and evaluation cost precious time. Only the essential testing should be performed. No one can predict when a tumor will transition from curable by surgery to incurable. Since this transition moment is not knowable, but we know it occurs, then a diagnosed or suspected lung cancer should be treated as an urgent matter. Non-essential testing and consultations should be avoided. Appointments should be scheduled promptly and arrangements should not include long waiting periods.
Secondly, it makes it understandable how some patients can be cured of metastatic cancer with chemotherapy given before or after surgery. The micrometastatic disease present in some of these patients is more amenable to treatment than the established, macroscopic metastatic disease of Stage IV patients, which is usually incurable by chemotherapy or any other means.
Surgery for lung cancer
The forgoing discussion makes it clear that surgery should be highly prioritized for all patients with operable lung cancer. All surgeons are not created equal. It is common in my practice to see patients that have been turned down for potentially life-saving surgery by less experienced, or less engaged surgeons. Some surgeons just don’t want to take on challenging cases. I see it all the time in my daily practice. These surgeons believe they are making the “right” choice for their patients, of course, when they turn a patient down for lung cancer surgery but the implications are quite serious for the patient nevertheless.
In fact, far more opportunity for cure has been lost at the hands of timid, overly selective surgeons in the treatment of lung cancer than problems encountered during surgery or afterward. To put this into perspective, the risk of an average patient not surviving after undergoing a lobectomy for lung cancer in 2015 should be 1% or less. So even a fairly high risk patient undergoing lobectomy is probably looking at a risk of death no higher than 10%. Why would a surgeon not offer surgery to such a patient? I honestly can’t answer this question but I know it is fact of life in lung cancer surgery that many surgeons won’t offer an operation to even those patients with a slightly higher risk than average.
Many patients ask “How do I know if the surgeon is experienced in lung cancer surgery?” One way to find out is to ask what kinds of different operations the surgeon does on a daily basis. If the surgeon answers that he or she performs cardiac surgery AND lung cancer surgery then they are probably part time lung cancer surgeons at best. The answer you want to hear is “Thoracic surgery only” or “Thoracic cancer surgery only”. Part time lung cancer surgeons are more likely to turn down patients for resection than dedicated lung cancer surgeons.
What are the risk of surgery?
When I assess a patient for surgery I am looking primarily at the risk the patient brings because of their overall health. Secondly, I assess the probability of complete removal of their tumor. In my experience, it is a rare patient that is too sick to survive lung cancer surgery. More important is the extent and stage of the patient’s tumor.
What are the problems that can occur following surgery? There are many potential problems that can arise, but fortunately, the most serious complications are rare. Pneumonia is by far the most common, serious problem and fortunately is usually successfully treated with antibiotics. Atrial fibrillation, or a fast heart and irregular heart beat occurs in 20-30% of lung surgery patients, and is usually not life threatening, and is treatable in most cases with medication. A variety of other problems can occur as well including urinary retention, blood clots, kidney failure, and even more rarely, heart attack and stroke. There are problems related to the surgery itself such as bleeding requiring transfusion or return to the operating room. Other reasons that may require a second operation would include poorly healing lung or air pipes that leak air; collections of fluid that require drainage; and infections that localize inside the chest.
Because of the improvements in the accuracy of imaging now, the risk of unexpectedly not being able to remove a tumor in the operating room is 1% or less.
Who should be a candidate for surgery?
Traditionally only patients in Stages I and II were considered for surgery. In my practice, and other experienced surgeons hands, patients with removable Stage III cancer will also be considered. In addition, there are some patients who have been treated with radiation instead of surgery but end up having their tumor come back. These patients can frequently have successful surgery. In addition, up to 10% of patients who have already had surgery for lung cancer can survive long enough to get a second cancer and should be evaluated for surgery if possible.


